A controlled selective synthesis of dihydropyrans through tandem reaction of alkynes with diazo compounds†
Abstract
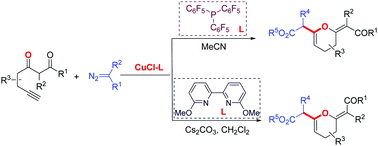
We report here an effective protocol to prepare highly functionalized dihydropyrans via sequential reaction of cross-coupling-intramolecular Michael addition of functionalized terminal alkynes and diazo compounds under mild reaction conditions. Importantly, by choosing different ligands, the configuration of exocyclic double bonds of dihydropyrans can be selectively controlled.


 Please wait while we load your content...
Please wait while we load your content...