Epitope imprinting enhanced IMAC (EI-IMAC) for highly selective purification of His-tagged protein
Abstract
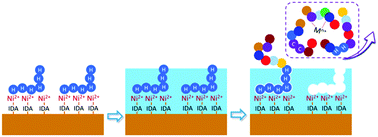
Recombinant protein technology occupies an important position in fields including biopharmaceutics, proteomics, structural and functional biology. However, the purification of His-tagged protein, the majority portion of recombinant protein, is seriously hindered by impurities. These impurities, including host proteins with inherent cysteine and histidine-rich regions or metal centers, are usually beyond the purification ability of commonly used IMAC materials. To remove this barrier, a novel purification material was developed through enhancing the selectivity of IMAC by means of surface epitope imprinting using His-tag, the common terminal of His-tagged protein, as the template. Characterizations including TEM, thermogravimetric analysis, X-ray photoelectron spectroscopy, measurement of DLS size and zeta potential were carried out to prove the fabrication of the imprinted shell. Results exhibited a high imprinting factor of 7.1. Besides, the adsorption kinetics were not affected by the surface imprinted shell and could reach adsorption equilibrium within 15 min. Compared with the substrate IMAC, the novel epitope imprinting enhanced IMAC (EI-IMAC) showed an obvious improvement (5% increase of purity) in the selectivity of His-tagged recombinant protein from crude cell lysis.
- This article is part of the themed collection: 2016 Journal of Materials Chemistry B Hot Papers

 Please wait while we load your content...
Please wait while we load your content...