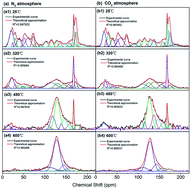
The effect of pyrolysis on the chemical characteristics of Antibiotic Fermentation Residue (AFR) was studied in this paper. Electron Spin Resonance (ESR) spectrometry, X-ray photoelectron spectroscopy (XPS) and solid state 13C nuclear magnetic resonance (13C NMR) spectroscopy were applied to investigate the variation of free radicals and carbon structures. Results indicated that the free radical concentration (Ng) of AFR/chars changed dramatically during the pyrolysis process, first increasing and then decreasing. Ng reached 1.24 × 1019 spins per g in a N2 atmosphere and 1.11 × 1019 spins per g in a CO2 atmosphere at 320 °C. The g-values monotonically decreased, demonstrating that the chemical structures of free radicals changed during pyrolysis. Bond cleavage in methoxyl groups, aliphatic C–O bonds, aliphatic C–C bonds and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups was enhanced with increasing pyrolysis temperature. These carbon structures converted to aromatic C–O bonds and aromatic C–C bonds through polymerization and condensation reactions. Compared with the CO2 atmosphere, the N2 atmosphere was more conducive to the production of free radicals and aromatization of carbon structures in chars during pyrolysis process.
O groups was enhanced with increasing pyrolysis temperature. These carbon structures converted to aromatic C–O bonds and aromatic C–C bonds through polymerization and condensation reactions. Compared with the CO2 atmosphere, the N2 atmosphere was more conducive to the production of free radicals and aromatization of carbon structures in chars during pyrolysis process.