Analysis of the solution conformations of T4 lysozyme by paramagnetic NMR spectroscopy†
Abstract
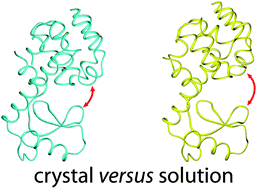
A large number of crystal structures of bacteriophage T4 lysozyme (T4-L) have shown that it contains two subdomains, which can arrange in a compact conformation (closed state) or, in mutants of T4-L, more extended structures (open state). In solution, wild-type T4-L displays only a single set of nuclear magnetic resonance (NMR) signals, masking any conformational heterogeneity. To probe the conformational space of T4-L, we generated a site-specific lanthanide binding site by attaching 4-mercaptomethyl dipicolinic acid via a disulfide bond to Cys44 in the triple-mutant C54T/C97A/S44C of T4-L and measured pseudocontact shifts (PCS) and magnetically induced residual dipolar couplings (RDC). The data indicate that, in solution and in the absence of substrate, the structure of T4-L is on average more open than suggested by the closed conformation of the crystal structure of wild-type T4-L. A slightly improved fit was obtained by assuming a population-weighted two-state model involving an even more open conformation and the closed state, but paramagnetic relaxation enhancements measured with Gd3+ argue against such a conformational equilibrium. The fit could not be improved by including a third conformation picked from the hundreds of crystal structures available for T4-L mutants.
- This article is part of the themed collection: Exploring the conformational heterogeneity of biomolecules: theory and experiments

 Please wait while we load your content...
Please wait while we load your content...