Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways†
Abstract
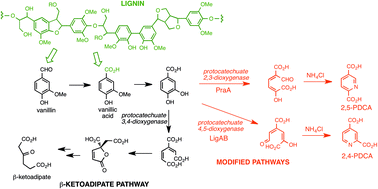
The heteropolymer lignin represents an untapped resource for production of renewable aromatic chemicals, if efficient depolymerisation methods can be developed. In this work, the metabolic pathways in Rhodococcus jostii RHA1 for degradation of aromatic lignin breakdown products are re-routed, in order to generate an aromatic dicarboxylic acid product that could be used for bioplastic synthesis. Protocatechuic acid is normally metabolised via ortho-cleavage to the β-keto-adipate pathway. Insertion of recombinant genes for protocatechuate 4,5-dioxygenase or protocatechuate 2,3-dioxygenase into R. jostii RHA1, followed by ammonia cyclisation of the extradiol cleavage products, generates pyridine 2,4-dicarboxylic acid or pyridine 2,5-dicarboxylic acid bioproducts in yields of 80–125 mg L−1 when grown on minimal media containing 1% wheat straw lignocellulose.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...