Reversible crosslinking of lignin via the furan–maleimide Diels–Alder reaction†
Abstract
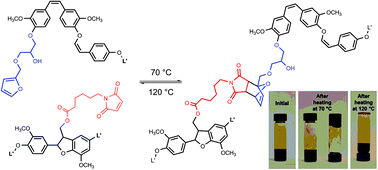
Two distinct functionalization schemes for Kraft lignin (KL) were developed to selectively incorporate furan and/or maleimide motifs as chain ends. The incorporation of furan functionalities was carried out by the selective and quantitative reaction of the lignin's phenolic OH groups with furfuryl glycidyl ether (FGE). Maleimide groups were introduced by esterifying the lignin's aliphatic and phenolic OH groups with 6 maleimidohexanoic acid (6-MHA), offering a high loading despite a somewhat incomplete conversion. Furan- and maleimide-functionalized lignins were subsequently combined to generate crosslinking via the Diels–Alder (DA) [4 + 2] cycloaddition reaction. The formation of the DA adduct was confirmed by 1H NMR. Under appropriate conditions, the formation of a gel was apparent, which turned back into the liquid state after performing the corresponding retro-DA reaction upon heating to 120 °C. This study reveals the significant versatility and potential of the developed strategy for the utilization of lignin-based recyclable networks.
- This article is part of the themed collection: Lignin chemistry and valorisation

 Please wait while we load your content...
Please wait while we load your content...