Low-temperature aqueous-phase reforming of ethanol on bimetallic PdZn catalysts†
Abstract
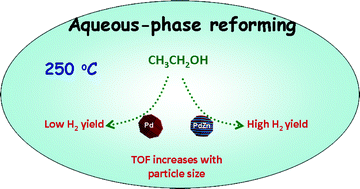
Bimetallic PdZn catalysts supported on carbon black (CB) and carbon nanotubes (CNTs) were found to be selective for CO-free H2 production from ethanol at low temperature (250 °C). On Pd, the H2 yield was low (~0.3 mol H2/mol ethanol reacted) and the CH4/CO2 ratio was high (~1.7). Addition of Zn to Pd formed the intermetallic PdZnβ phase (atomic ratio of Zn to Pd is 1) with increased H2 yield (~1.9 mol H2/mol ethanol reacted) and CH4/CO2 ratio of <1. The higher H2 yield and low CH4 formation was related to the improved dehydrogenation activity of the L10 PdZnβ phase. The TOF increased with particle size and the CNTs provided the most active and selective catalysts, which may be ascribed to pore-confinement effects. Furthermore, no significant changes in either the supports or the PdZnβ particles was found after aqueous-phase reforming (APR) indicating that the metal nanoparticles and the carbon support are hydrothermally stable in the aqueous phase at elevated temperatures and pressures (>200 °C, 65 bar). No CO was detected for all the catalysts performed in aqueous-phase reaction, indicating that both monometallic Pd and bimetallic PdZn catalysts have high water-gas shift activity during APR. However, the yield of H2 is considerably lower than the theoretical value of 6 H2 per mole ethanol which is due to the presence of oxygenated products and methane on the PdZn catalysts.
- This article is part of the themed collection: Catalysis in the USA

 Please wait while we load your content...
Please wait while we load your content...