Electron transfer within a reaction path model calibrated by constrained DFT calculations: application to mixed-valence organic compounds†
Abstract
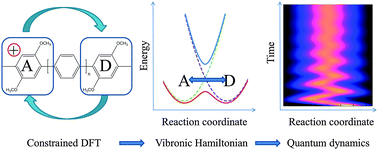
The quantum dynamics of electron transfer in mixed-valence organic compounds is investigated using a reaction path model calibrated by constrained density functional theory (cDFT). Constrained DFT is used to define diabatic states relevant for describing the electron transfer, to obtain equilibrium structures for each of these states and to estimate the electronic coupling between them. The harmonic analysis at the diabatic minima yields normal modes forming the dissipative bath coupled to the electronic states. In order to decrease the system-bath coupling, an effective one dimensional vibronic Hamiltonian is constructed by partitioning the modes into a linear reaction path which connects both equilibrium positions and a set of secondary vibrational modes, coupled to this reaction coordinate. Using this vibronic model Hamiltonian, dissipative quantum dynamics is carried out using Redfield theory, based on a spectral density which is determined from the cDFT results. In a first benchmark case, the model is applied to a series of mixed-valence organic compounds formed by two 1,4-dimethoxy-3-methylphenylene fragments linked by an increasing number of phenylene bridges. This allows us to examine the coherent electron transfer in extreme situations leading to a ground adiabatic state with or without a barrier and therefore to the trapping of the charge or to an easy delocalization.
- This article is part of the themed collection: Measurement and prediction of quantum coherence effects in biological processes

 Please wait while we load your content...
Please wait while we load your content...