Selective synthesis of secondary benzylic (Z)-allylboronates by Fe-catalyzed 1,4-hydroboration of 1-aryl-substituted 1,3-dienes†‡
Abstract
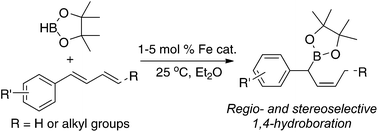
We have prepared and characterized a series of new iminopyridine iron complexes with a bulky diphenylphosphinomethyl-ketimine substituent. Using one of these iron complexes as the precatalyst, the hydroboration of 1-substituted 1,3-dienes containing aromatic groups with pinacolborane occurs regio- and stereoselectively to form secondary (Z)-allylboronates. In addition, we report the first examples of Suzuki–Miyaura cross-coupling of secondary allylboronates with aryl bromides. The reactions catalyzed by Pd(dba)2/Ad2PnBu yield the coupling products with excellent regioselectivity (γ/α > 99 : 1) and E-selectivity of the olefin geometry (E/Z > 99 : 1).

 Please wait while we load your content...
Please wait while we load your content...