Controllable mono-/di-alkenylation of aryl alkyl thioethers tuned by oxidants via Pd-catalysis†
Abstract
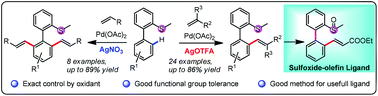
Oxidant-controlled selective mono-/di-alkenylation of aryl C–H bonds via Pd-catalysis is reported. The substrate scopes for both the mono- and di-alkenylation were good. Thioether was used as the directing group and the product can be transformed to a useful sulfoxide-olefin ligand by simple oxidation in quantitative yield.

 Please wait while we load your content...
Please wait while we load your content...