Crystalline nanoparticle aggregation in non-aqueous solvents†
Abstract
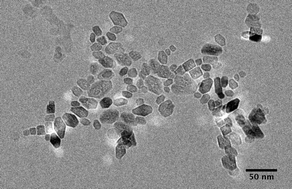
The aggregation of suspended metal oxide and oxyhydroxide nanoparticles dramatically changes as a function of solvent properties. In addition, solvent selection can offer an additional parameter for controlling aggregation and aggregative crystal growth (e.g., oriented aggregation). Here, results are presented demonstrating dramatic changes in the aggregation state of goethite (α-FeOOH), anatase (TiO2), and ferrihydrite (Fe5HO8·4H2O) nanoparticles as a function of changing solvents. Results are discussed in the context of Derjaguin and Landau, Verwey and Overbeek (DLVO) theory, additional dynamic light scattering data, and solvent properties like dielectric constant and coordinative ability. Generally speaking, solvents that strongly coordinate the metal oxide/oxyhydroxide surfaces produce the smallest and least compact aggregates, whereas those that weakly coordinate produce the largest and most compact aggregates. Dielectric constant and the nanoparticle morphology are also important factors that impact aggregation state.
- This article is part of the themed collection: Nanocrystal growth via oriented attachment

 Please wait while we load your content...
Please wait while we load your content...