Kinetics of crystal growth of nanogoethite in aqueous solutions containing nitrate and sulfate anions†
Abstract
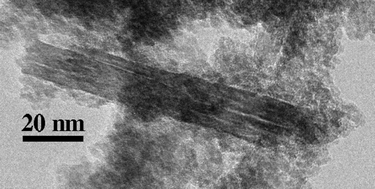
Iron oxyhydroxide nanoparticles, relatively abundant and highly reactive components of most natural ecosystems, are known to grow via the oriented attachment (OA)-based crystal growth pathway. Here, we conducted experiments in highly alkaline solutions containing sulfate and nitrate anions to test the differential impact of these anions on the various steps in this growth pathway. Nanogoethite (α-FeOOH) was prepared by reacting ferric sulfate or ferric nitrate with potassium hydroxide, and aging at room temperature, 50, 60 and 70 °C. X-ray diffraction (XRD) was used to confirm the synthesis of goethite and Rietveld analyses were used to determine the particle sizes in samples with different aging times. Particle morphology and microstructure of some samples were investigated using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). TEM images confirmed growth of goethite nanorods via OA. OA growth kinetics were modeled using a previously reported kinetic equation. An Arrhenius plot indicated the activation energy for OA-based crystal growth in the sulfate system was 45.2 kJ mol−1, higher than in the nitrate system (23.1 kJ mol−1). The magnitude of the activation energy suggests that the rate-limiting step for OA growth is diffusion of nanoparticles in solution, a first step necessary for particle–particle attachment. We attribute the larger effect of sulfate compared to nitrate on the activation energy to greater structuring of water by sulfate. The kinetic pre-exponential factor for the sulfate system is much higher than that for the nitrate system, and results in faster growth kinetics at high temperature. Sulfate may accelerate the dehydration of the interface, a subsequent step required for OA-based growth, possibly through increasing the rate of water exchange at the nanoparticle surfaces. These findings motivate conduction of new experiments to further probe the kinetics of individual steps in OA-based crystal growth, suggest routes to inhibit or promote OA relative to atom-by-atom growth pathways, and provide clues to how variation in solution chemistry could impact mineral growth in natural systems.
- This article is part of the themed collection: Nanocrystal growth via oriented attachment

 Please wait while we load your content...
Please wait while we load your content...