Selenite and tellurite form mixed seleno- and tellurotrisulfides with CstR from Staphylococcus aureus†
Abstract
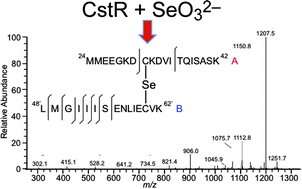
Staphylococcus aureus CstR (CsoR-like sulfur transferase repressor) is a member of the CsoR family of transition metal sensing metalloregulatory 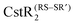 and
and 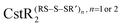 , respectively. Here, we investigate if CstR performs similar chemistry with related
, respectively. Here, we investigate if CstR performs similar chemistry with related 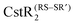 and
and 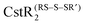 . The modification of Cys31 also drives an allosteric switch that negatively regulates DNA binding while
. The modification of Cys31 also drives an allosteric switch that negatively regulates DNA binding while
- This article is part of the themed collection: Microbial Metallomics

 Please wait while we load your content...
Please wait while we load your content...