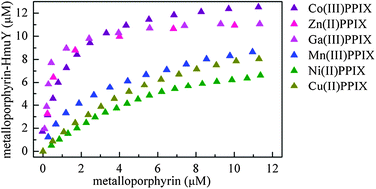
Porphyromonas gingivalis, a major etiological agent of chronic periodontitis, acquires haem from host haemoproteins through a haem transporter HmuR and a haemophore HmuY. The aim of this study was to analyse the binding specificity of HmuY towards non-iron metalloporphyrins which may be employed as antimicrobials to treat periodontitis. HmuY binds gallium(III), zinc(II), cobalt(III), manganese(III), nickel(II), and copper(II) protoporphyrin IX but in a manner different to iron(III) protoporphyrin IX which uses His134 and His166 as axial ligands. The metal ions in Ga(III)PPIX and Zn(II)PPIX can accept only His166 as an axial ligand, whereas nickel(II) and copper(II) interact exclusively with His134. Two forms of pentacoordinate manganese(III) are present in the Mn(III)PPIX–HmuY complex since the metal accepts either His134 or His166 as a single axial ligand. The cobalt ion is hexacoordinate in the Co(III)PPIX–HmuY complex and binds His134 and His166 as axial ligands; however, some differences in their environments exist. Despite different coordination modes of the central metal ion, gallium(III), zinc(II), cobalt(III), and manganese(III) protoporphyrin IX bound to the HmuY haemophore cannot be displaced by excess haem. All of the metalloporphyrins examined bind to a P. gingivalis wild-type strain with higher ability compared to a mutant strain lacking a functional hmuY gene, thus corroborating binding of non-iron metalloporphyrins to purified HmuY protein. Our results further clarify the basis of metalloporphyrin acquisition by P. gingivalis and add to understanding of the interactions with porphyrin derivatives which exhibit antimicrobial activity against P. gingivalis.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...