Ketonization of enols in aqueous solution: is carbon protonation always rate-determining?†
Abstract
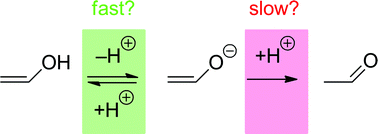
The pH–rate profiles for the ketonization of the (E)- and (Z)-photoenols of
- This article is part of the themed collection: In honour of Professor Kurt Schaffner

 Please wait while we load your content...
Please wait while we load your content...