Doubly charged protonated a ions derived from small peptides†
Abstract
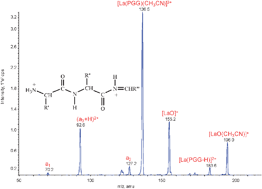
Protonated a2 and a3 (therefore doubly charged) ions in which both charges lie on the peptide backbone are formed in collision-induced dissociations of [LaIII(peptide)(CH3CN)m]3+ complexes. Abundant (a3+H)2+ ions are formed from triproline (PPP) and
- This article is part of the themed collection: Multiply charged ions (MCIs) in the gas-phase

 Please wait while we load your content...
Please wait while we load your content...