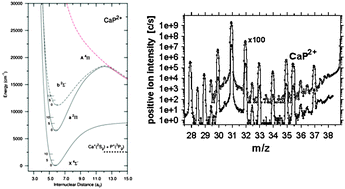
Sputtering (ion surface bombardment) of various calcium-containing powder samples with an energetic (17 keV), high-current 16O− beam has produced the diatomic dications of CaSi2+, CaP2+, CaF2+, CaH2+, CaCl2+, CaBr2+ and CaI2+. These molecular gas-phase species have been identified in positive ion mass spectra at half-integer m/z values; their ion flight times through a magnetic-sector mass spectrometer were roughly 10−5 s. Most of them appear to be novel molecular ions; the stability of the latter four (CaH2+, CaCl2+, CaBr2+ and CaI2+) had been demonstrated in previous theoretical studies, whereas only CaF2+ and CaBr2+ had been observed before. Here we combine the results of our experimental search with a detailed theoretical study of the remaining three systems CaSi2+, CaP2+ and CaF2+. All electronic states correlating with the first dissociation channel are characterized using high level ab initio electronic structure calculations. In their ground states, we find CaSi2+ to be a long-lived metastable molecule, whereas CaF2+ and CaP2+ are thermodynamically stable, with respective equilibrium internuclear distances of 6.253, 4.740, and 5.731 a0. CaSi2+ has a well depth of 7116 (0.88) cm−1 (eV) and a dissociation asymptote 7956 (0.99) cm−1 (eV) below the ground state minimum. The dissociation energy of CaF2+ is estimated to be 3404 (0.42) cm−1 (eV), whereas for CaP2+ we found 2547 (0.32) cm−1 (eV), and a barrier height of 8118 (1.01) cm−1 (eV). Their adiabatic double ionisation energies are 22.87, 16.91, and 17.32 eV, respectively, for the F, Si, and P containing dications.

 Please wait while we load your content...
Please wait while we load your content...