Efficient capture of strontium from aqueous solutions using graphene oxide–hydroxyapatite nanocomposites†
Abstract
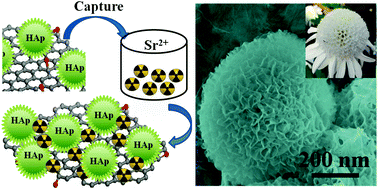
Three-dimensional hierarchical flower-like graphene oxide–hydroxyapatite (GO–HAp) nanocomposites were synthesized by a simple biomimetic method in a modified simulated body fluid (mSBF). The obtained GO–HAp nanocomposites were characterized by field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transformed infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), Raman spectroscopy, and N2 adsorption–desorption analysis. The formation mechanism was proposed and the prepared GO–HAp was applied as an adsorbent to remove strontium from large volumes of aqueous solutions. A maximum adsorption capacity of 702.18 mg g−1 was achieved on GO–HAp, almost two fold higher than that of bare HAp and nine fold higher than that of GO. The effects of pH, adsorbent content, contact time and Sr2+ initial concentrations on Sr2+ removal from solution by GO–HAp were systematically investigated, and the results indicated that the removal of Sr2+ by GO–HAp was weakly dependent on solution pH. The results herein reveal that the GO–HAp nanocomposites had exceptional potential as a suitable material for preconcentration and solidification of radiostrontium from large volumes of aqueous solutions in nuclear waste management and radiostrontium pollution cleanup.
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...