Linking Pd(ii) and Ru(ii) phthalocyanines to single-walled carbon nanotubes†
Abstract
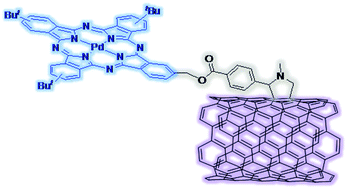
The preparation of new Pc-SWCNT hybrid materials is described. The synthesis of both Pd(II)Pc-SWCNT 7 and Ru(II)bis(pyridine)Pc-SWCNT 9 was carried out by esterification reaction between modified SWCNTs and the corresponding unsymmetric Pcs 1 and 2, both endowed with three solubilizing tert-butyl groups and a hydroxymethyl function. Compound 1 was prepared following the straightforward, statistical condensation of the corresponding phthalonitriles. However the preparation of Ru(II)bis(pyridine)Pc 2 required a multistep procedure relying on consecutive cyclotetramerization, protection, metallation and deprotection reactions. Modified SWCNT 6 was prepared, as previously described, by the Prato reaction between HiPCo nanotubes, N-methylglycine and 4-formylbenzoic acid. The COOH-containing material 6 was successfully reacted with Pd(II)Pc 1 to give Pd(II)Pc-SWCNT 7, which was fully characterized by different techniques. However, the incorporation of Ru(II)bis(pyridine)Pc 1 did not take place when applying the above-mentioned conditions. The preparation of SWCNT 8 endowed with 4-carboxyphenyl moieties was found to be essential to covalently link Ru(bis(pyridine))Pc 1 to the nanotube material by ester bond formation. Although spectroscopic characterization supports the covalent binding of Pc molecules to the modified SWCNT sidewalls, direct evidence of the presence of Ru(II) in the hybrid material could not be obtained.
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...