A copper single-atom catalyst towards efficient and durable oxygen reduction for fuel cells†
Abstract
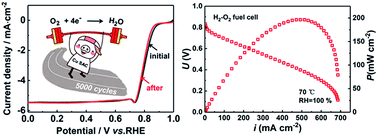
Single-atom catalysts (SACs) of transition metals have attracted enormous attention as replacements of Pt-based catalysts towards the oxygen reduction reaction (ORR). Even though Cu is nature's choice for ORR catalysis in many forms of life from bacteria to humans; however, only a few studies of Cu-based SACs for the ORR have been reported. Furthermore, the applications of Cu SACs for fuel cells have rarely been demonstrated. In this work, we prepared a Cu SAC via a simple pyrolysis method using Cu phthalocyanine as the precursor. The catalyst shows a half-wave potential of 0.81 V (vs. RHE) in alkaline medium towards the ORR, which is 30 mV lower than that of commercial Pt/C (20 wt%). It also shows high durability with only 9 mV negative shift of half-wave potential after 5000 cycles in alkaline medium. An anion exchange membrane fuel cell based on the Cu SAC exhibits a peak power density of 196 mW cm−2 at 70 °C. The density functional theory calculation suggests that the transformation process from OOH* to O* is the rate determining step over the Cu SAC. The Cu SAC could be a promising non-noble ORR catalyst for fuel cell applications.


 Please wait while we load your content...
Please wait while we load your content...