A novel nickel metal–organic framework with fluorite-like structure: gas adsorption properties and catalytic activity in Knoevenagel condensation†
Abstract
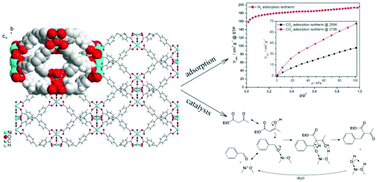
A new non-interpenetrating 3D metal–organic framework {[Ni4(μ6-MTB)2(μ2-H2O)4(H2O)4]·10DMF·11H2O}n (DMF = N,N′-dimethylformamide) built from nickel(II) ions as connectors and methanetetrabenzoate ligands (MTB4−) as linkers has been synthesized and characterized. The single crystal X-ray diffraction showed that complex exhibits CaF2-like fluorite structure topology and four types of 3D channels with sizes about 12.6 × 9.4 Å2, 9.4 × 8.0 Å2, 12.6 × 11.7 Å2 and 14.9 × 14.9 Å2, which are filled with guest molecules. Conditions of the activation of the compound have been studied and optimized by powder X-ray diffraction during in situ heating, thermogravimetric analysis and infrared spectroscopy. Nitrogen and carbon dioxide adsorption showed that the activated sample exhibits a BET specific surface area of 700 m2 g−1 and a carbon dioxide uptake of 12.36 wt% at 0 °C, which are the highest values reported for the compounds of the MTB4− series. The complex was tested in Knoevenagel condensation of aldehydes and active methylene compounds. Straightforward dependence of the substrate conversion on the size of used aldehyde was established. A possible mechanism of Knoevenagel condensation over a MTB4− containing a metal–organic framework was proposed.

 Please wait while we load your content...
Please wait while we load your content...