Removal of uranium(vi) from aqueous solution using iminodiacetic acid derivative functionalized SBA-15 as adsorbents
Abstract
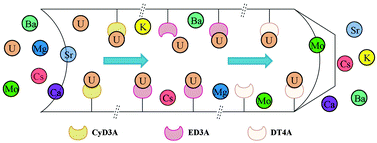
Three different functional SBA-15 were prepared by a post-grafting method using three iminodiacetic acid derivatives of ethylenediaminetriacetic acid (ED3A), diethylenetriaminetetraacetic acid (DT4A), and 1,2-cyclohexylenedinitrilotriacetic acid (CyD3A), which were used as adsorbents for removal of uranium(VI) from aqueous solution. These materials were characterized by FT-IR, NMR, TEM, nitrogen adsorption/desorption experiments, and elemental analysis. The effect of pH, ionic strength, contact time, solid–liquid ratio, initial metal ion concentration, temperature, and coexisting ions on uranium(VI) sorption behaviors of the functionalized SBA-15 was studied. Typical sorption isotherms (Langmuir and Freundlich) were determined for the sorption process, and the maximum sorption capacity was calculated. The influence of functional groups on uranium(VI) sorption was also discussed. As a result, compared with other current U(VI) sorbents (granite, kaolin, attapulgite), SBA-15-1,2-cyclohexylenedinitrilotriacetic acid (SBA-15-CyD3A) possessed good selective sorption properties, which had potential application in separation of uranium(VI).

 Please wait while we load your content...
Please wait while we load your content...