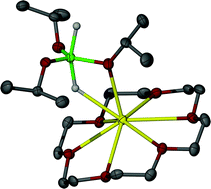
A range of hydridosilicate anions has been prepared and characterised by spectroscopic, structural and computational methods. The general approach involved reaction of KH with a neutral silane precursor in the presence of [18]crown-6. In this manner, [K([18]crown-6)]+ salts of [Ph3SiH2]– (1), [Ph3SiF2]– (9), and [(p-FC6H4)3SiHF]–/[(p-FC6H4)3SiH2]– (12) were stabilised and characterized by NMR spectroscopy and X-ray diffraction. In each case, the anion adopts a trigonal bipyramidal (TBP) geometry with three equatorial phenyl groups eclipsing the axial Si–H/Si–F bonds. The Si–H⋯K distances, along with DFT calculations on 1, indicate an electrostatic interaction that does not dictate the geometry adopted by the anion. A [H2SiOiPr3]– salt (7) has also been crystallised in the same way; X-ray diffraction shows in this case a distorted TBP array with axial hydride ligands, and both Si–H⋯K and Si–O⋯K interactions. 1H NMR exchange experiments show 1 to undergo facile hydride exchange with Ph3SiH. Compound 1 acts as a good hydride transfer reagent to a variety of substrates, but its high reactivity often results in redistribution and other side reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...