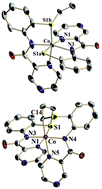
Anaerobic reaction of Co(O2CMe)2·4H2O with the thioether-containing acyclic pyrazine amide hexadentate ligand 1,4-bis[o-(pyrazine-2-carboxamidophenyl)]-1,4-dithiobutane (H2L11) (–CH2CH2– spacer between the two pyrazine amide tridentate coordination units) furnishes [CoII(L11)]·MeOH (1a) having CoN2(pyrazine)N′2(amide)S2(thioether) coordination. It exhibits an eight-line EPR spectrum, attesting to a low-spin (S = 1/2) state of CoII. A similar reaction in air, however, furnishes [CoIII(L3a3a)(L3b3b)]·2MeOH (2a) (S = 0), resulting from a C–S bond cleavage reaction triggered by an acetate ion as a base, having CoN2(pyrazine)N′2(amide)S(thioether)S′(thiolate) coordination. On the other hand, the reaction of Co(O2CMe)2·4H2O with 1,4-bis[o-(pyrazine-2-carboxamidophenyl)]-1,5-dithiopentane (H2L22) (–CH2CH2CH2– spacer between the two pyrazine amide tridentate coordination units) in air affords a cobalt(II) complex [CoII(L22)]·MeOH (1b·MeOH) (S = 1/2); its structurally characterized variety has the composition 1b·C6H6. Interestingly, 1b·MeOH undergoes facile metal-centred oxidation by aerial O2–H2O2–[Fe(η5-C5H5)2][PF6], which led to the isolation of the corresponding cobalt(III) complex [CoIII(L22)][ClO4] (2b). When treated with methanolic KOH, 2b affords a low-spin (S = 0) organocobalt(III) complex [CoIII((L2′2′)] (3). Structures of all complexes, except 1a, have been authenticated by X-ray crystallography. A five-membered chelate-ring forming ligand L11(2–) effects C–S bond cleavage and a six-membered chelate-ring forming ligand L22(2–) gives rise to Co–C bond formation, in cobalt(III)-coordinated thioether functions due to α C–H bond activation by the base. A rationale has been provided for the observed difference in the reactivity properties. The spectroscopic properties of the complexes have also been investigated. Cyclic voltammetry experiments in MeCN–CH2Cl2 reveal facile metal-centred reversible-to-quasireversible CoIV–CoIII (or a ligand-centred redox process; 2a), CoIII–CoII (1a, 1b·MeOH, 2a, 2b and 3), CoII–CoI (1a, 1b·MeOH, 2a and 2b), and CoI–Co0 (1a, 1b·MeOH and 2b) redox processes.

 Please wait while we load your content...
Please wait while we load your content...