Visible-light-induced radical cascade reaction to prepare oxindoles via alkyl radical addition to N-arylacryl amides†
Abstract
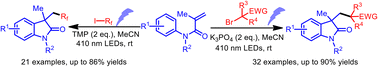
Herein we describe a photochemical approach towards oxindoles by the radical cascade reaction of α,β-unsaturated N-arylacryl amides with alkyl bromides or iodides upon visible light irradiation. This transition metal- and photosensitizer-free protocol afforded diverse oxindoles with C3 quaternary centers in high product yields under mild reaction conditions. Importantly, the method was applicable to prepare the core skeletons of (±)-physovenine, (±)-esermethole and (±)-physostigmine.


 Please wait while we load your content...
Please wait while we load your content...