Palladium-catalyzed regiodivergent arylamination/aryloxygenation of allenamide†
Abstract
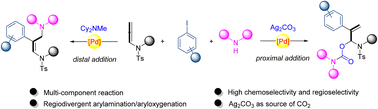
A palladium-catalyzed multi-component regiodivergent arylamination/aryloxygenation of allenamide is reported. The bases are critical to the chemoselectivity and regioselectivity: (1) Cy2NMe as a base could lead to distal addition that generated a 2,1-arylamination product; (2) Ag2CO3 as a base could result in proximal addition for a 2,3-aryloxygenation product via a four-component transformation, including an unexpected CO2 insertion from Ag2CO3. Notably, the corresponding alkenes formed in 2,3-arylamination could be presented with excellent Z configuration, which was modulated via the steric hindrance of a coordinated palladium intermediate. Two possible π-allyl-Pd complexes were proposed for the resulting regioselectivity: the neutral η3 π-allyl-Pd complex favored the γ substitution where there was less steric hindrance; cationic η1 π-allyl-Pd complex generated via the coordination of Ag favored the reductive elimination at the allylic position adjacent to nitrogen.


 Please wait while we load your content...
Please wait while we load your content...