A facile two-step synthesis of 8-arylated guanosine mono- and triphosphates (8-aryl GXPs)
Abstract
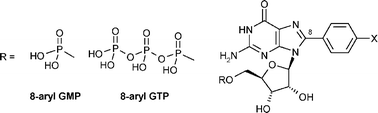
We report a simple and high-yielding two-step procedure for the preparation of 8-arylated guanosine mono- and triphosphates (8-aryl GXPs). The key step of our synthesis is the Suzuki–Miyaura coupling of unprotected 8-bromo

 Please wait while we load your content...
Please wait while we load your content...