Valence and coordination form transition of tungsten ions in molten alkali chlorides†
Abstract
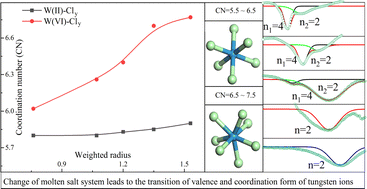
This study examined the possibility of deep significance for the reduction of low-valence tungsten to inhibit disproportionation reactions in various molten alkali chlorides. Electrolysis and electrochemical tests of tungsten carbide were carried out in molten LiCl, LiCl–KCl, NaCl–KCl, NaCl–CsCl, and KCl–CsCl. One finding was that the reduction valence of tungsten ions decreased as the radius of the solvent alkali ion increased. This phenomenon may be viewed from the dissolution of tungsten carbide and the existence and deposition of tungsten ions. The mechanism of tungsten ion reduction and the stable configuration of tungsten ion groups were confirmed via a detailed study of the computational calculation. The increase in the radius of the solvent alkali ion was conducive to the dissolution of tungsten from tungsten carbide in the form of low valence state. Other results also indicated that W(II) ion groups first deposited on the cathode. They had the advantages of smaller coordination numbers and faster diffusion combined. Morphological and composition analysis results of the products are also presented.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...