Synthesis of non-hydrolysable mimics of glycosylphosphatidylinositol (GPI) anchors†
Abstract
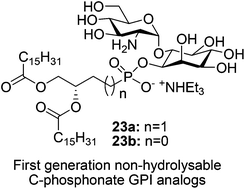
Synthesis of first generation non-hydrolysable C-phosphonate GPI analogs, viz., 6-O-(2-amino-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol-1-O-(sn-3,4-bis(palmitoyloxy)butyl-1-phosphonate) 23a and 6-O-(2-amino-2-deoxy-α-D-glucopyranosyl)-D-myo-inositol-1-O-(sn-2,3-bis(palmitoyloxy)propyl-1-phosphonate) 23b, is reported. The target compounds were synthesized by the coupling of α-pseudodisaccharide 21 with phosphonic acids 18a and 18b respectively in quantitative yield followed by de-protection. These synthetic C-phosphonate GPI-probes were resistant to phosphatidylinositol specific phospholipase C (PI-PLC) and also showed moderate inhibition of the enzyme activity.

 Please wait while we load your content...
Please wait while we load your content...