Properties of 5- and/or 2-modified 2′-O-cyanoethyl uridine residue: 2′-O-cyanoethyl-5-propynyl-2-thiouridine as an efficient duplex stabilizing component†
Abstract
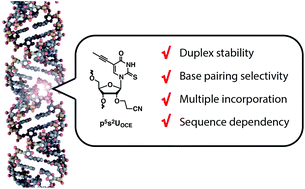
We systematically synthesized eight types of 5- and/or 2-modified uridine derivatives and evaluated their effect on duplex stability. The incorporation of 2′-O-cyanoethyl-2-thio-5-propynyluridine (p5s2UOCE) into RNA was significantly effective for stabilization of RNA/RNA (+8.5 °C) and DNA/RNA (+10.4 °C) duplexes. These striking effects were maintained in oligonucleotides with different sequences or multiple incorporations. In addition, p5s2UOCE increased selectivity toward the correct AU Watson–Crick base pair over the most stable mismatched base pair in both RNA/RNA and DNA/RNA duplexes. Hence, p5s2UOCE could be useful for various applications of modified oligonucleotides that need high duplex stability and base pairing selectivity.

 Please wait while we load your content...
Please wait while we load your content...