Carbon nanodots prepared from o-phenylenediamine for sensing of Cu2+ ions in cells†
Abstract
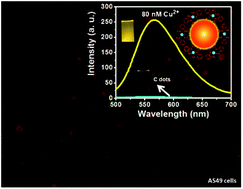
A simple hydrothermal method was applied to prepare carbon nanodots (C dots) from o-phenylenediamine (OPD). The C dots exhibit photoluminescence at 567 nm when excited at 420 nm. In the presence of Cu2+ ions, the colour of C dots changes from yellow to orange, with an increased PL intensity as a result of the formation of Cu(OPD)2 complexes on the surfaces of C dots. The D-band to G-band ratios of C dots in the absence and presence of 80 nM Cu2+ ions are 1.31 and 4.75, respectively. The C dots allow the detection of Cu2+ ions with linearity over a concentration range of 2–80 nM, with a limit of detection of 1.8 nM at a signal-to-noise ratio of 3. The cell viability values of A549, MCF-10A, and MDA-MB-231 cells treated with 3 μg mL−1 of C dots are all greater than 99%, showing their great biocompatibility. Having great water dispersibility, photostability, chemical stability (against NaCl up to 0.5 M), great selectivity, and biocompatibility, the C dots have been employed for the localization of Cu2+ ions in the cancer cells (A549 cells) treated with 10 μM Cu2+ ions.

 Please wait while we load your content...
Please wait while we load your content...