Nickel-catalyzed reductive coupling of unactivated alkyl bromides and aliphatic aldehydes†
Abstract
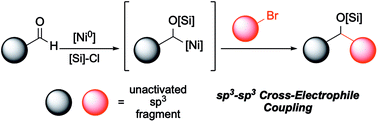
A mild, convenient coupling of aliphatic aldehydes and unactivated alkyl bromides has been developed. The catalytic system features the use of a common Ni(II) precatalyst and a readily available bioxazoline ligand and affords silyl-protected secondary alcohols. The reaction is operationally simple, utilizing Mn as a stoichiometric reductant, and tolerates a wide range of functional groups. The use of 1,5-hexadiene as an additive is an important reaction parameter that provides significant benefits in yield optimizations. Initial mechanistic experiments support a mechanism featuring an alpha-silyloxy Ni species that undergoes formal oxidative addition to the alkyl bromide via a reductive cross-coupling pathway.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...