Catalytic enantioselective synthesis of 1,4-dihydropyridines via the addition of C(1)-ammonium enolates to pyridinium salts†
Abstract
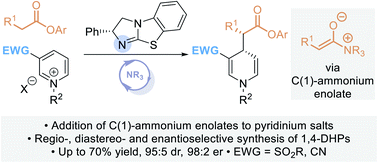
The regio- and stereoselective addition of C(1)-ammonium enolates – generated in situ from aryl esters and the isothiourea catalyst (R)-BTM – to pyridinium salts bearing an electron withdrawing substituent in the 3-position allows the synthesis of a range of enantioenriched 1,4-dihydropyridines. This represents the first organocatalytic approach to pyridine dearomatisation using pronucleophiles at the carboxylic acid oxidation level. Optimisation studies revealed a significant solvent dependency upon product enantioselectivity, with only toluene providing significant asymmetric induction. Using DABCO as a base also proved beneficial for product enantioselectivity, while investigations into the nature of the counterion showed that co-ordinating bromide or chloride substrates led to higher product er than the corresponding tetrafluoroborate or hexafluorophosphate. The scope and limitations of this process are developed, with enantioselective addition to 3-cyano- or 3-sulfonylpyridinium salts giving the corresponding 1,4-dihydropyridines (15 examples, up to 95 : 5 dr and 98 : 2 er).
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...