Copper-catalyzed divergent construction of naphthofurans and benzochromanes from 2-naphthols, 4-methylene-quinazolin(thi)ones, and N,N-dimethylethanolamine†
Abstract
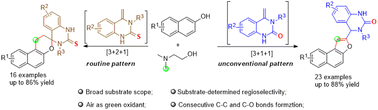
We report herein an unprecedented Cu(II)-catalyzed divergent synthesis of naphthofurans and benzochromanes from 2-naphthols, N,N-dimethylethanolamine, and methylene-3,4-dihydroquinazolin-2(1H)-ones (or methylene-3,4-dihydroquinazolin-2(1H)-thiones) under mild conditions. With methylene-3,4-dihydroquinazolin-2(1H)-ones, naphthofuran products were afforded through an unconventional [3 + 1 + 1] cyclization while benzochromanes were obtained via a routine [3 + 2 + 1] annulation when methylene-3,4-dihydroquinazolin-2(1H)-thiones were applied as substrates. Both products could be obtained via a one-pot/two-step process starting from 2-aminoacetophenone with isocyanates or isothiocyanates.


 Please wait while we load your content...
Please wait while we load your content...