A zuranolone nanocrystal formulation enables solubility-independent in vivo study of pentylenetetrazol-induced seizures in a rat model
Abstract
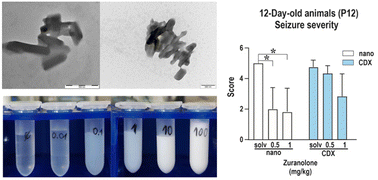
Neuroactive steroids are a promising class of substances with many potential therapeutic applications, but their preclinical evaluation is challenging due to very low aqueous solubility. A common practice is to “solubilise” such drugs using water-miscible solvents, but this approach has drawbacks: the drug can precipitate uncontrollably after injection, the solvent can artificially increase membrane permeability, and such formulations are not directly transferrable to humans. It would be beneficial to use the same physical form of the drug during preclinical and clinical studies. This work reports an approach based on an aqueous suspension of phospholipid-coated nanocrystals of zuranolone, chosen as a representative of poorly soluble neuroactive steroid drugs. The wet stirred media milling method was used for creating a nanosuspension with a mean particle size of d1,0 = 114 ± 39 nm, colloidally stable in PBS over 24 months at a concentration up to 100 mg mL−1. The applicability of the nanosuspension was demonstrated in a study of pentylenetetrazol-induced seizures in developing rats as a model of human generalized tonic–clonic seizures. The incidence and severity of seizures were assessed for the zuranolone nanosuspension and compared to an established dosage as a cyclodextrin complex. The incidence of generalized seizures with or without the tonic phase was found to be lower in P12 rats receiving zuranolone in doses of 0.5 and 1 mg kg−1 in the nanocrystal formulation than in those receiving the cyclodextrin solution. In contrast, both formulations significantly decreased seizure severity in P25 rats at a dose of 1 mg kg−1. Crucially, the nanocrystal formulation enabled the creation of a concentration series independent of the thermodynamic solubility of the drug. A constant volume appropriate to the body size of the young rats could therefore be injected during the in vivo study.


 Please wait while we load your content...
Please wait while we load your content...