Dual ligand approach increases functional group tolerance in the Pd-catalysed C–H arylation of N-heterocyclic pharmaceuticals†
Abstract
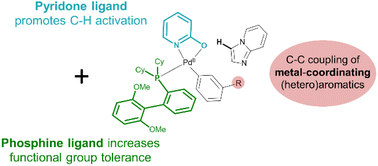
The excellent functional group tolerance of the Suzuki–Miyaura cross-coupling reactions has been decisive for their success in the pharmaceutical industry. Highly diversified (hetero)aromatic scaffolds can be effectively coupled in the final step(s) of a convergent synthetic route. In contrast, electrophilic Pd catalysts for non-directed C–H activation are particularly sensitive to inhibition by coordinating groups in pharmaceutical precursors. While C–H arylation enables the direct conversion of (hetero)aromatics without preinstalled functional or directing groups, its functional group tolerance should be increased to be viable in late-stage cross-couplings. In this work, we report on a dual ligand approach that combines a strongly coordinating phosphine ligand with a chelating 2-hydroxypyridine for the highly robust C–H coupling of bicyclic N-heteroaromatics with aryl bromide scaffolds. The catalyst speciation was studied via in situ XAS measurements, confirming the coordination of both ligands under the reaction conditions. The C–H activation catalyst was shown to be tolerant to a wide range of pharmaceutically relevant scaffolds, including examples of late-stage functionalization of known drug molecules.


 Please wait while we load your content...
Please wait while we load your content...