Synthesis of quinoxaline derivatives via aromatic nucleophilic substitution of hydrogen†
Abstract
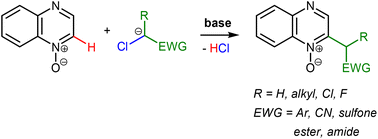
The electrophilic nature of quinoxaline has been explored in the vicarious nucleophilic substitution (VNS) of hydrogen with various carbanions as nucleophiles in an attempt to develop a general method for functionalizing the heterocyclic ring. Only poorly stabilized nitrile carbanions were found to give the VNS products. 2-Chloroquinoxaline gave products of SNAr of chlorine preferentially. A variety of quinoxaline derivatives containing cyanoalkyl, sulfonylalkyl, benzyl or ester substituents, including fluorinated ones, have been prepared in the VNS reactions with quinoxaline N-oxide.


 Please wait while we load your content...
Please wait while we load your content...