Easy access to α-carbonyl sulfones using cross-coupling of α-aryl-α-diazoesters with sulfonyl hydrazides†
Abstract
A facile synthesis of α-carbonyl sulfones has been accomplished by the cross-coupling of α-aryl-α-diazoesters with sulfonyl hydrazides in the presence of CuI and DBU. The reaction employs inexpensive and bench stable sulfonyl hydrazides as a sulfonyl source, and facilitates the migratory insertion with α-aryl-α-diazoesters under mild reaction conditions.
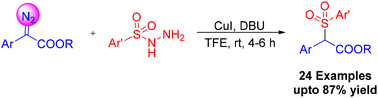


 Please wait while we load your content...
Please wait while we load your content...