Linking polynitro hexaazaisowurtzitane cages via an N,N'-methylene bridge: a promising strategy for designing energetic ensembles of CL-20 derivatives and adjusting their properties†
Abstract
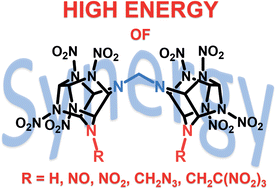
To solve actual tasks for implementation of the modern space program, it is necessary to search for new high-energy components of rocket propellants. For this purpose, we designed a novel family of highly energetic N,N′-methylene bridged polynitro hexaazaisowurtzitane materials bearing secondary amino, N-nitro, N-nitroso, N-(2,2,2-trinitroethyl), and N-azidomethyl terminal groups, and developed an efficient approach to their synthesis via condensation of the corresponding N-methoxymethyl precursors and targeted energetic functionalization. All the new compounds were fully characterized using high-resolution mass spectrometry, IR and multinuclear NMR (1H, 13C{1H}, 14N, and 15N) spectroscopy, and with single-crystal X-ray diffraction. For these substances, the enthalpies of formation were determined experimentally via combustion calorimetry. Thermal stability measurements and safety testing were carried out. Their energetic potential as components of rocket propellants was studied using high-temperature chemical equilibrium thermodynamic calculations. The materials of this new series exhibit high density (up to 1.95 g cm−3) and heat resistance values (decomposition onset up to 226 °C). Moreover, they have a very high enthalpy of formation (up to ∼2 MJ kg−1, which is 2-fold that of CL-20) and reduced friction sensitivity (around 100 N lower than the friction sensitivity of CL-20). These remarkable comprehensive characteristics make these materials promising for use in energetic compositions. The target compounds are highly effective energetic components for metal-free rocket propellants that provide, with moderate organic contents, specific impulse values up to 5.5–9.5 s higher than for similar formulations based on CL-20.


 Please wait while we load your content...
Please wait while we load your content...