Effect of connectivity variation in azulene-BODIPY triads and their optoelectronic properties†
Abstract
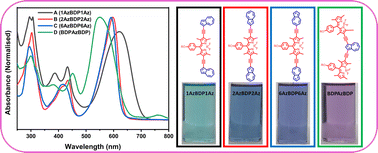
Azulene is a non-alternant non-benzenoid aromatic hydrocarbon with an inherent dipole moment of 1.08 D providing azulene-containing conjugated systems with a narrow HOMO–LUMO gap. Here we discuss the design, synthesis and characterization of a series of azulene-BODIPY triads (A, B, C, and D) with acetylene spacer by varying the linking position of azulene. The molecular triad A involving connectivity of azulene via 1-position shows the most extended conjugation consistent with the electron-rich nature of 1,3-positions of azulene. The most extended conjugation of A is also supported by its largest reduction onset potential of −0.87 V compared to the triads B, C, and D. The molecules display excellent thermal stability, and theoretical results indicate that the polarity change of organic FETs based on the isomeric triads could be controlled by the molecular orbital distributions through the connection position between the azulene unit and BODIPY.


 Please wait while we load your content...
Please wait while we load your content...