First representatives of C-glycosyl 1,2,4,5-tetrazines: synthesis of 3-β-d-glucopyranosyl 1,2,4,5-tetrazines and their transformation into 3-β-d-glucopyranosyl pyridazines†
Abstract
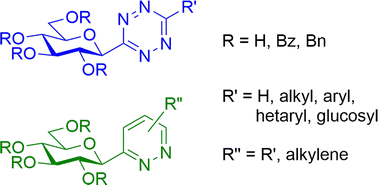
1,2,4,5-Tetrazines (s-tetrazines) are a long known class of compounds with many applications e.g. in heterocyclic syntheses and recently in bioorthogonal chemistry. C-Glycopyranosyl tetrazines are unknown in the literature, therefore, we have started to study their synthesis. In this paper ring closing reactions leading to s-tetrazines have been investigated with suitable β-D-glucopyranosyl precursors and the feasible transformations have been identified. In addition, the obtained C-glucopyranosyl tetrazines’ basic protecting group compatibility and their utility in inverse electron demand Diels–Alder cycloadditions towards a variety of C-glucopyranosyl pyridazines have been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...