Lithium-mediated electrochemical dinitrogen reduction reaction
Abstract
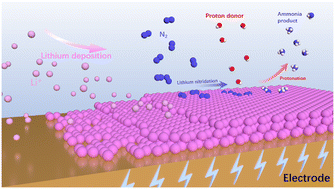
The Haber–Bosch process is the dominant approach for NH3 production today, but the process has to be maintained at energy-intensive high temperatures and pressures. Li-mediated electrocatalytic dinitrogen reduction reaction (eN2RR) could instead enable sustainable and green NH3 production at ambient conditions. Lithium mediators realize the synthesis of NH3via the formation of Li3N, and thus lower the energy required for the direct cleavage of N2. There has now been a surge of interest in devising approaches to optimize the NH3 yield rate and faradaic efficiency of the eN2RR process by employing different catalysts as well as electrolytes. This review discusses the recent advances in the field of the Li-mediated eN2RR along with the latest insights into the proposed catalytic mechanisms. Moreover, it also highlights the state-of-the-art reported electrocatalysts and electrolytes that have revolutionized the field of the Li-mediated eN2RR. In addition to the above, our review provides a critical overview of certain limitations and a future prospectus that will provide a way forward to explore this area.
Keywords: Nitrogen reduction reaction; Ammonia; Electrocatalysis; Lithium; Reaction mechanism.
- This article is part of the themed collections: Virtual Collection—Electrocatalysis, Virtual Collections—ICM HOT Papers, Virtual Collections—ICM Reviews and Energy Frontiers: Electrochemistry and Electrochemical Engineering


 Please wait while we load your content...
Please wait while we load your content...