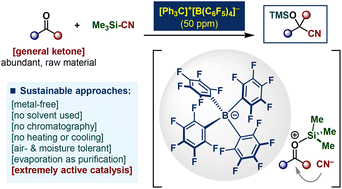
Sustainable organocatalytic cyanosilylation of ketones by PPM-level loading of triphenylcarbenium tetrakis(pentafluorophenyl)borate†
Abstract
Along with the significant demand for sustainable homogeneous catalysis, the importance of efficient synthesis and simple purification of complex organic compounds is attracting attention. Herein, we report the cyanosilylation of ketones using tritylium tetrakis(pentafluorophenyl)borate ([Ph3C]+[B(C6F5)4]−) as an extremely active organocatalyst. The neutral, air- and moisture-tolerant nature of the solid salt is beneficial for user-friendly handling. With this transition metal-free catalytic system, the corresponding cyanohydrin trimethylsilyl ethers could be obtained with a 50 ppm [= 0.005 mol%] catalyst loading by employing a wide variety of ketones as starting materials. Under solvent-free conditions with neither temperature manipulation (cooling or heating) nor column chromatography, the gram-scale reaction works perfectly, and highly pure products can be obtained by removing the volatiles. Experimental and analytical studies support in situ generated silylium with a weakly coordinating anion ion-pair as an active catalyst playing a crucial role in excellent reactivity.


 Please wait while we load your content...
Please wait while we load your content...