Electrochemical oxidative cascade cyclization of olefinic amides and alcohols leading to the synthesis of alkoxylated 4H-3,1-benzoxazines and indolines†
Abstract
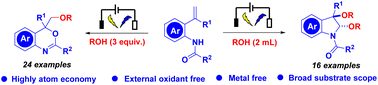
Synthesis technologies can be used directly and selectively to construct two different heterocycles from one substrate. Herein, we introduce a mild and efficient electrochemical oxidative strategy to construct alkoxylated 4H-3,1-benzoxazines and indolines. High atom efficiency and transition metal- and oxidant-free conditions are the striking features of this protocol. In a simple undivided cell, various olefinic amides and alcohols react to generate 40 examples of benzoxazines and indolines. This radical cascade reaction provides a facile method for constructing C–O bonds in one step. Furthermore, most of the compounds exhibit good inhibitory activity on tumour cell lines.


 Please wait while we load your content...
Please wait while we load your content...