Poly(ester-co-ether) from ring-opening copolymerisation of sustainable 2-methyltetrahydrofuran with β-butyrolactone†
Abstract
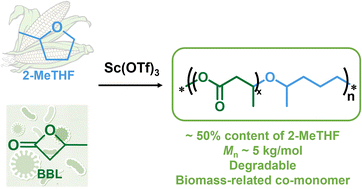
2-Methyltetrahydrofuran (2-MeTHF) is a biomass-based tetrahydrofuran derivative that is considerably difficult to polymerise. This contribution reports Sc(OTf)3-catalysed cationic ring-opening copolymerization of 2-MeTHF with β-butyrolactone (BBL), another renewable monomer. The possibility of copolymerization is verified by density functional theory (DFT) calculations. The obtained copolymer poly(2-MeTHF-co-BBL) has a number-average molecular weight (Mn) of 5.2 kg mol−1, in which the 2-MeTHF content is 48.8 mol% in accordance with the initial feed ratio of 50 mol%. The copolymers have been characterized by NMR, SEC, MALDI-TOF MS and DSC measurements. The cationic ring-opening polymerisation mechanism is confirmed by kinetic studies and DFT calculations. Poly(2-MeTHF-co-BBL) is able to degrade completely within 24 h under alkaline conditions, and has potential as an environmentally friendly material from biomass sources.


 Please wait while we load your content...
Please wait while we load your content...