Selective hydrogenolysis of bio-renewable tetrahydrofurfurylamine to piperidine on ReOx-modified Rh catalysts†
Abstract
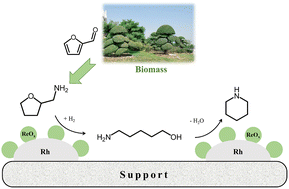
Piperidine is an important cyclic amine with versatile applications. However, its commercial process is not green and depends mainly on the hydrogenation of fossil resource-based pyridine. Here, we report a novel one-pot approach to the sustainable synthesis of piperidine from bio-renewable tetrahydrofurfurylamine (THFAM) via its hydrogenolysis to 5-amino-1-pentanol (APO) and the subsequent intramolecular amination of APO. SiO2-supported Rh–ReOx catalysts exhibited high efficiency and stability in the THFAM reaction to piperidine, providing a high yield of 91.5% at 200 °C and 2.0 MPa H2 in water. Such high efficiency of Rh–ReOx/SiO2 was found to be related to the synergistic effect between the Rh nanoparticles and ReOx species on the kinetically-relevant cleavage of the C–O bond neighboring the C–NH2 group in THFAM, involving the strong adsorption of the C–NH2 group on ReOx and the heterolytic dissociation of H2 on Rh. This work provides an efficient strategy for the selective cleavage of the C–O bonds neighboring the C–NH2 groups, irrespective of their presence in amino ethers or amino alcohols, and the green production of piperidine and its derivatives from biomass resources.


 Please wait while we load your content...
Please wait while we load your content...