Calcium carbide residue – a promising hidden source of hydrogen†
Abstract
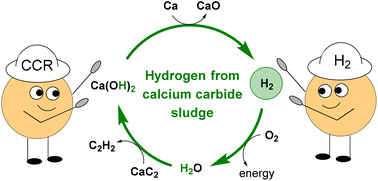
Hydrogen is a key industrial product used as a “green energy” carrier and fully compatible co-reagent in many crucial transformations in the chemical industry. Hydrogen production is based on hydrocarbons or water splitting. Both sources are in demand and can find many other uses. Here, calcium carbide residue (CCR) – sludge after acetylene production from calcium carbide – was used for hydrogen generation. The CCR was mixed with various metals, such as zinc, magnesium, iron and calcium, and heated up to 500–700 °C. As a result, hydrogen evolution was observed. According to volumetric data, the yield of hydrogen was up to 99%. The solid residue after hydrogen release was a mixture of the corresponding metal oxides. According to powder XRD, SEM, and EDX analyses of the inorganic residue, when calcium metal was used, only calcium oxide – a starting component in calcium carbide production – was formed. Thus, the waste CCR can be revalorised and used as a hydrogen source followed by re-use in carbide synthesis. The retrieved hydrogen from the CCR was used in the hydrogenation reaction, and the desired products were obtained with up to 99% yields, clearly confirming the nature of the evaluated gas. When calcium carbide was hydrolysed with deuterium oxide, deuterated CCR was obtained. Sintering of CCR-d2 with calcium metal resulted in D2-gas evolution, which was further used in the synthesis of D-labeled compounds with up to 75% deuterium incorporation.


 Please wait while we load your content...
Please wait while we load your content...