Preparation and characterization of an iron–β-cyclodextrin inclusion complex: factors influencing the host–guest interaction†
Abstract
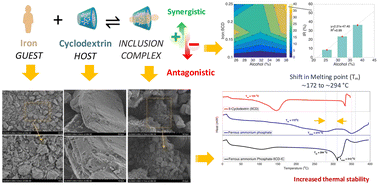
Cyclodextrins have received attention recently due to their superior binding with countless hydrophobic molecules. The host–guest interaction between the cyclodextrin cavity and the hydrophobic component not only facilitates the formation of a strong inclusion complex (IC), but also improves its stability against thermal degradation. The functionality of cyclodextrins for the delivery of hydrophilic components is less explored in comparison. This study discusses the application of β-cyclodextrin (βCD) for the delivery of highly bioavailable and hydrophilic iron, ferric sodium EDTA, which exhibits great functionality in the presence of polyphenols and phytates with potential application in food fortification. The formation of IC was dependent on the cyclodextrin amount and alcoholic co-solvent and was influenced by the stirring duration. For ferric sodium EDTA, the highest inclusion rate (IR) of ∼77% was obtained after 72 hours of mixing in 25.4% (v/v) alcohol at a ratio of iron : βCD of 1 : 6. A higher IR (∼96%) was obtained after 6 hours of stirring with less soluble ferrous ammonium phosphate in comparison. The melting temperature (Tm) of the ferrous ammonium phosphate complex increased from ∼172 to ∼294 °C. The high IR and enhanced thermal resistance of the complex make βCDs potential carriers for ferrous ammonium phosphate delivery and fortification of foods processed at high temperatures.


 Please wait while we load your content...
Please wait while we load your content...