Group 3 dialkyl complexes of a rigid monoanionic NNN-donor pincer ligand: synthesis, structures, unexpected reactivity with CPh3+, and hydroamination catalysis†
Abstract
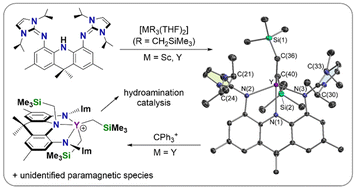
Palladium-catalyzed coupling of 4,5-dibromo-2,7,9,9-tetramethylacridan with two equivalents of 1,3-diisopropylimidazolin-2-imine afforded 4,5-bis(1,3-diisopropylimidazolin-2-imino)-2,7,9,9-tetramethylacridan, H[AII2]. Reaction of the H[AII2] pro-ligand with one equivalent of [M(CH2SiMe3)3(THF)2] (M = Y or Sc) yielded the base-free neutral dialkyl complexes [(AII2)M(CH2SiMe3)2] {M = Y (1) and Sc (2)}. The rigid AII2 pincer ligand affords a similar steric profile to the previously reported XA2 pincer ligand, but is monoanionic rather than dianionic. Reaction of 1 with one equiv. of [CPh3][B(C6F5)4] in C6D5Br generated a highly active catalyst for intramolecular alkene hydroamination. However, rather than forming the expected monoalkyl cation, this reaction afforded a diamagnetic product which was identified as [(AII2-CH2SiMe3)Y(CH2SiMe3)2][B(C6F5)4] (3; AII2-CH2SiMe3 is a neutral tridentate ligand with a central amine donor flanked by imidazolin-2-imine groups) in approx. 20% yield, accompanied by HCPh3 (∼2 equiv. relative to 3), an unidentified paramagnetic product (detected by EPR spectroscopy), and a small amount of colourless precipitate. The unexpected reactivity of 1 with CPh3+ is thought to involve initial AII2 ligand backbone oxidation, given that the zwitterionic form of the ligand contains a phenylene ring with two adjacent anionic nitrogen donors, similar to a redox-non-innocent, dianionic ortho-phenylenediamido ligand.


 Please wait while we load your content...
Please wait while we load your content...