Cyclic(alkyl)(amino)carbene ruthenium complexes for Z-stereoselective (asymmetric) olefin metathesis†‡
Abstract
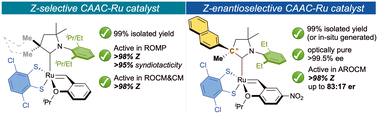
The first Z-stereoselective catechodithiolate ruthenium complexes containing cyclic(alkyl)(amino)carbene ligands are reported. Isolated in nearly quantitative yields or in situ generated, these catalysts demonstrated remarkable Z selectivity (Z/E ratio up to >98/2) in ring-opening metathesis polymerization (ROMP), ring-opening-cross metathesis (ROCM) and cross-metathesis (CM). Thanks to the efficient chiral HPLC resolution of racemic CAAC-complex precursors, optically pure dithiolated complexes were also synthesized allowing to produce enantioenriched Z-ROCM products in >99/1 Z/E with good levels of enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...